Difference between revisions of "Unit 1: Biochemistry"
| Line 55: | Line 55: | ||
Hydrocarbon= Molecule of only hydrogen and carbon. | Hydrocarbon= Molecule of only hydrogen and carbon. | ||
| − | Macromolecule= Very large molecule. These include carbohydrates, proteins, and nucleic acids. | + | Macromolecule= Very large molecule. These include [[carbohydrates]], proteins, and nucleic acids. |
Polymer= Long molecule consisting of many similar or identical building blocks linked by covalent bonds. | Polymer= Long molecule consisting of many similar or identical building blocks linked by covalent bonds. | ||
Revision as of 10:58, 16 October 2020
Vocab:
Matter = Anything that takes up space and has mass.
Element = Substance that cannot be broken down into other substances by chemical reactions.
Essential element = Elements required for an organism to live and reproduce. Hydrogen, oxygen, carbon, nitrogen are the main essential elements. There are also some trace elements.
Compound = substance consisting of 2 or more different elements.
Molecule = substance consisting of 2 or more elements. Not necessarily different.
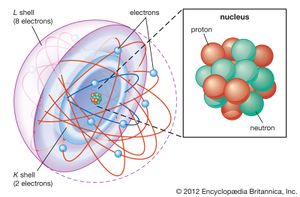
Atom = Smallest unit of matter. Still retains the properties of an element.
Subatomic particles = two nuclear subatomic particles: Proton (+tive charge) and neutrons (no charge); and 1 orbiting subatomic particle: Electron (-tive charge).
atomic number= number of protons.
atomic mass= Average weight of number of protons plus neutrons.
isotope= Different atomic forms of the same element (different number of neutrons).
Valance shell= outer shell of electrons. Bonding part of an atom. Either 2 (first) or 8 (all others) electrons.
Chemical bond= sharing or taking of electrons.
covalent bond= sharing a pair of two valence electrons by two atoms. It is the strongest bond.
Ionic bond= Atom taking an electron from another atom. Forms between a cation and a anion. Weak bond in aqueous solutions.
Electronegativity = The power of an atom to attract electrons to itself.
Non polar bond= Electrons are shared equally.
Polar bond= Electrons are not shared equally.
Anion= negative ion.
Cation= positive ion.
Hydrogen bond= Bond between hydrogen and an electronegative atom. See high specific heat.
Acid= Substance that increases the hydrogen ion concentration of a solution.
Base= Substance that reduces the hydrogen ion concentration of a solution.
Molecular mass= the molecular mass of a molecule is equal to the sum of the atomic masses of it's elements.
Avogadro's number= 6.02*10^23
Molar mass= a molecule's molar mass is equal to the molecule's molecular mass number in grams. This is called 1 mole. It is also equal in mass to an Avogadro's number amount of the molecule.
Organic chemistry = Study of compounds containing carbon.
Hydrocarbon= Molecule of only hydrogen and carbon.
Macromolecule= Very large molecule. These include carbohydrates, proteins, and nucleic acids.
Polymer= Long molecule consisting of many similar or identical building blocks linked by covalent bonds.
Monomer= The similar or identical building block used in a polymer.
Enzyme= Specialised macromolecules that speed up chemical reactions.