Difference between revisions of "Unit 1: Biochemistry"
(vocab and concept) |
|||
| Line 1: | Line 1: | ||
| − | + | '''Vocab''': | |
| − | + | Matter = Anything that takes up space and has mass. | |
| − | + | Element = Substance that cannot be broken down into other substances by chemical reactions. | |
| − | + | Essential element = Elements required for an organism to live and reproduce. Hydrogen, oxygen, carbon, nitrogen are the main essential elements. There are also some trace elements. | |
| − | Atom = | + | Compound = substance consisting of 2 or more '''different''' elements. |
| + | |||
| + | Molecule = substance consisting of 2 or more elements. Not necessarily different. | ||
| + | [[File:Shell-atomic-model-shell-shells-electrons-energy.jpg|thumb|Atom diagram]] | ||
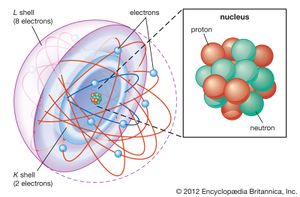
| + | Atom = Smallest unit of matter. Still retains the properties of an element. | ||
Subatomic particles = two nuclear subatomic particles: Proton (+tive charge) and neutrons (no charge); and 1 orbiting subatomic particle: Electron (-tive charge). | Subatomic particles = two nuclear subatomic particles: Proton (+tive charge) and neutrons (no charge); and 1 orbiting subatomic particle: Electron (-tive charge). | ||
| + | |||
| + | atomic number= number of protons. | ||
| + | |||
| + | atomic mass= Average weight of number of protons plus neutrons. | ||
| + | |||
| + | isotope= Different atomic forms of the same element (different number of neutrons). | ||
| + | |||
| + | Valance shell= outer shell of electrons. Bonding part of an atom. Either 2 (first) or 8 (all others) electrons. | ||
| + | |||
| + | Chemical bond= sharing or taking of electrons. | ||
| + | |||
| + | covalent bond= sharing a pair of two valence electrons by two atoms. It is the strongest bond. | ||
| + | |||
| + | Ionic bond= Atom taking an electron from another atom. Forms between a cation and a anion. Weak bond in aqueous solutions. | ||
| + | |||
| + | Electronegativity = The power of an atom to attract electrons to itself. | ||
| + | |||
| + | Non polar bond= Electrons are shared equally. | ||
| + | |||
| + | Polar bond= Electrons are '''not''' shared equally. | ||
| + | |||
| + | Anion= negative ion. | ||
| + | |||
| + | Cation= positive ion. | ||
| + | |||
| + | hydrogen bond= Bond between hydrogen and an electronegative atom. | ||
Revision as of 08:30, 15 October 2020
Vocab:
Matter = Anything that takes up space and has mass.
Element = Substance that cannot be broken down into other substances by chemical reactions.
Essential element = Elements required for an organism to live and reproduce. Hydrogen, oxygen, carbon, nitrogen are the main essential elements. There are also some trace elements.
Compound = substance consisting of 2 or more different elements.
Molecule = substance consisting of 2 or more elements. Not necessarily different.
Atom = Smallest unit of matter. Still retains the properties of an element.
Subatomic particles = two nuclear subatomic particles: Proton (+tive charge) and neutrons (no charge); and 1 orbiting subatomic particle: Electron (-tive charge).
atomic number= number of protons.
atomic mass= Average weight of number of protons plus neutrons.
isotope= Different atomic forms of the same element (different number of neutrons).
Valance shell= outer shell of electrons. Bonding part of an atom. Either 2 (first) or 8 (all others) electrons.
Chemical bond= sharing or taking of electrons.
covalent bond= sharing a pair of two valence electrons by two atoms. It is the strongest bond.
Ionic bond= Atom taking an electron from another atom. Forms between a cation and a anion. Weak bond in aqueous solutions.
Electronegativity = The power of an atom to attract electrons to itself.
Non polar bond= Electrons are shared equally.
Polar bond= Electrons are not shared equally.
Anion= negative ion.
Cation= positive ion.
hydrogen bond= Bond between hydrogen and an electronegative atom.